A BIOTECH veteran has hailed a Glasgow firm that claims to have discovered two separate potential treatments for Covid-19 patients for use before they are put on ventilators.
ILC Therapeutics has appointed Dr Alan Walker as chief executive to lead and streamline the development of the new treatments as it urgently seeks funding of £4 million to accelerate safety studies and clinical trials.
Mr Walker has over 50 years’ experience in the life science sector and is former chief executive of Internis and Ryboquin, also spending 28 years at Warner Lambert.
READ MORE: Shares in Scottish biotech firm rise as antibody test is CE-mark approved
He said: “It is remarkable that a small, biotech start-up of this size would have discovered not one but two novel treatment methods, and I want to help charter the course as we hopefully bring these treatments to clinical trials fast and work to save lives.
“There has been much talk within the scientific community of Interferon Alpha 2, but this is not an effective treatment method and frankly has stalled further interferon research by decades.
"ILCT’s Interferon Alpha 14 could prevent the need for patients to be put on ventilators by boosting their innate immune systems as the virus progresses."
READ MORE: Omega Diagnostics gears up for mass roll-out of virus testing kits
He added: “We have seen that few patients survive once they are put on ventilators, so the quicker we can develop this treatment in a safe and scalable way, the better.”
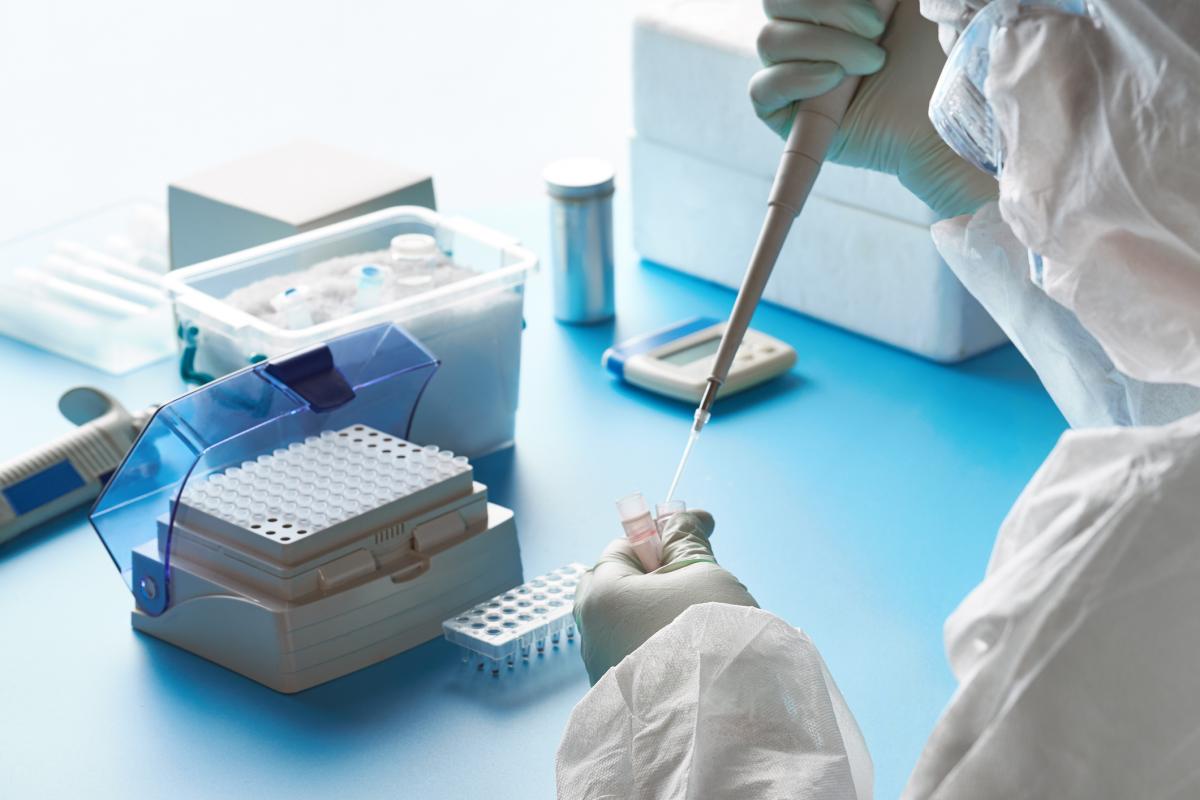
The firm has patented a new Interferon-Alpha subtype, called Interferon Alpha 14, which an be administered to patients through injection or inhalation.
It claims the natural human molecule treatment could prevent Covid-19 induced Acute Respiratory Distress Syndrome (ARDS), which would mean that a considerable number of patients may no longer need to be on a ventilator.
Mr Walker will be leading funding alongside chief scientific officer Professor Bill Stimson. The funding will allow for safety studies and the first clinical trials, in early 2021.
If these are successful, then the Alpha 14 could be accelerated quickly through the drug approval process.





Why are you making commenting on The Herald only available to subscribers?
It should have been a safe space for informed debate, somewhere for readers to discuss issues around the biggest stories of the day, but all too often the below the line comments on most websites have become bogged down by off-topic discussions and abuse.
heraldscotland.com is tackling this problem by allowing only subscribers to comment.
We are doing this to improve the experience for our loyal readers and we believe it will reduce the ability of trolls and troublemakers, who occasionally find their way onto our site, to abuse our journalists and readers. We also hope it will help the comments section fulfil its promise as a part of Scotland's conversation with itself.
We are lucky at The Herald. We are read by an informed, educated readership who can add their knowledge and insights to our stories.
That is invaluable.
We are making the subscriber-only change to support our valued readers, who tell us they don't want the site cluttered up with irrelevant comments, untruths and abuse.
In the past, the journalist’s job was to collect and distribute information to the audience. Technology means that readers can shape a discussion. We look forward to hearing from you on heraldscotland.com
Comments & Moderation
Readers’ comments: You are personally liable for the content of any comments you upload to this website, so please act responsibly. We do not pre-moderate or monitor readers’ comments appearing on our websites, but we do post-moderate in response to complaints we receive or otherwise when a potential problem comes to our attention. You can make a complaint by using the ‘report this post’ link . We may then apply our discretion under the user terms to amend or delete comments.
Post moderation is undertaken full-time 9am-6pm on weekdays, and on a part-time basis outwith those hours.
Read the rules hereLast Updated:
Report this comment Cancel