
Human trials of the coronavirus vaccine being developed by the University of Oxford and AstraZeneca have been put on hold.
The decision to halt the clinical trials come owing to a reported side effect in a patient in the UK.
AstraZeneca said it was investigating whether the patient’s reported side effect is connected with the vaccine.
But is this a setback for the development of the vaccine and what happens next? Here's everything you need to know.
– What is the vaccine?
The vaccine – called ChAdOx1 nCoV-19 – uses a weakened version of a common cold virus (adenovirus) which causes infections in chimpanzees.
It has been genetically changed so it is impossible for it to grow in humans.
It is hoped the vaccine will make the body recognise and develop an immune response to the spike protein – recognisable in images of the virus – that will help stop Covid-19 from entering human cells and therefore prevent infection.
– Why have trials been put on hold?
AstraZeneca issued a statement saying the late-stage studies of the vaccine had been paused while the company investigates whether the patient’s reported side effect was connected with the vaccine.
A spokeswoman said the pause was part of a standard review process which occurs in trials if there is a “potentially unexplained illness” reported in any trial subject, and that the subject’s illness could also be coincidental.
READ MORE: Coronavirus: How does the Oxford Covid-19 vaccine work?
 Doses of the Oxford Covid-19 vaccine (Sean Elias/University of Oxford)
Doses of the Oxford Covid-19 vaccine (Sean Elias/University of Oxford)
No details about the patient suffering the potential side effect, or the nature of the reaction, were given.
But The New York Times has reported that the patient had been diagnosed with transverse myelitis, an inflammatory syndrome that affects the spinal cord often sparked by viral infections.
– What happens next?
AstraZeneca said it had voluntarily paused vaccination to allow review of safety data by an independent committee to take place.
Wellcome Trust director and Sage member Professor Sir Jeremy Farrar told BBC Radio 4’s Today programme that such an occurrence is quite common but each one must be taken seriously.
He said an independent investigation would look at whether the illness in the trial volunteer is related to the vaccine or placebo and, if not, then the trial can restart safely.
Prof Farrar added that it is inevitable that some of the 30,000 to 40,000 people given the vaccine will have illnesses unrelated to it.
READ MORE: Coronavirus: Oxford University vaccine trial paused to investigate patient's side effect
– Is this a setback?
Temporary holds of large medical studies are not uncommon, and looking into any unexpected reactions is a mandatory part of safety testing.
It was not immediately clear how long AstraZeneca’s pause would last.
Health Secretary Matt Hancock said the Oxford vaccine trial had been paused earlier in the year.
He said: “There was a pause earlier in the summer and that was resolved without a problem.”
– How are the trials progressing?
The vaccine is being trialled in tens of thousands of volunteers in the UK, South Africa, Brazil and the US.
It is expected that there will be up to 50,000 participants globally, the University of Oxford has said.
This trial aims to assess how well people across a broad range of ages could be protected from Covid-19.
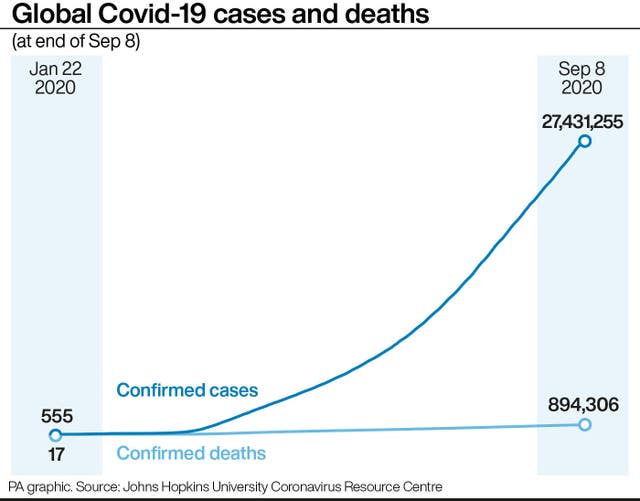 (PA Graphics)
(PA Graphics)
Results from the late-stage trials are anticipated later this year, the university has said.
– What did the preliminary results suggest?
The results of the clinical trials, published in The Lancet in July, indicate that the vaccine candidate has triggered two responses in the immune system.
READ MORE: Coronavirus: Inverness care home first to trial tracking technology
The first is that it stimulates the immune system to produce antibodies – proteins produced by the blood in response to antigens which are harmful substances that come from outside the body, such as from viruses or bacteria – and that it also causes the body to produce T-cells.
Oxford’s Covid-19 vaccine produces a good immune response, reveals new study. Teams at @VaccineTrials and @OxfordVacGroup have found there were no safety concerns, and the vaccine stimulated strong immune responses: https://t.co/krqRzXMh7B pic.twitter.com/Svd3MhCXWZ
— University of Oxford (@UniofOxford) July 20, 2020
If the non-specific immune cells which respond to any invader instantly cannot tackle it, the T-cells come into play.
These cells attack the virus directly.
With questions remaining about the duration of the antibody response to Covid-19, research suggests T-cells have a more important role in offering protection against the disease.
What has been said since trials were stopped?
An AstraZeneca spokeswoman said the pause was part of a standard review process which occurs in trial if there is a “potentially unexplained illness” reported in any trial subject, and that the subject’s illness could also be coincidental.
“As part of the ongoing randomised, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee,” the spokeswoman said in a statement.
“This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials.
“In large trials illnesses will happen by chance but must be independently reviewed to check this carefully.
“We are working to expedite the review of the single event to minimise any potential impact on the trial timeline. We are committed to the safety of our participants and the highest standards of conduct in our trials.”
– If a successful vaccine is developed, can it be manufactured to scale?
Production of the vaccine has already been scaled up ahead of the trial to prepare as early as possible for potential future deployment.
The Government has signed deals for 90 million doses of promising Covid-19 vaccines, with more in the pipeline.
The latest deal is for vaccines being developed by an alliance between the pharmaceutical giants BioNtech and Pfizer as well as the firm Valneva.
This is in addition to 100 million doses of a vaccine being developed by Oxford University with AstraZeneca.



Why are you making commenting on The Herald only available to subscribers?
It should have been a safe space for informed debate, somewhere for readers to discuss issues around the biggest stories of the day, but all too often the below the line comments on most websites have become bogged down by off-topic discussions and abuse.
heraldscotland.com is tackling this problem by allowing only subscribers to comment.
We are doing this to improve the experience for our loyal readers and we believe it will reduce the ability of trolls and troublemakers, who occasionally find their way onto our site, to abuse our journalists and readers. We also hope it will help the comments section fulfil its promise as a part of Scotland's conversation with itself.
We are lucky at The Herald. We are read by an informed, educated readership who can add their knowledge and insights to our stories.
That is invaluable.
We are making the subscriber-only change to support our valued readers, who tell us they don't want the site cluttered up with irrelevant comments, untruths and abuse.
In the past, the journalist’s job was to collect and distribute information to the audience. Technology means that readers can shape a discussion. We look forward to hearing from you on heraldscotland.com
Comments & Moderation
Readers’ comments: You are personally liable for the content of any comments you upload to this website, so please act responsibly. We do not pre-moderate or monitor readers’ comments appearing on our websites, but we do post-moderate in response to complaints we receive or otherwise when a potential problem comes to our attention. You can make a complaint by using the ‘report this post’ link . We may then apply our discretion under the user terms to amend or delete comments.
Post moderation is undertaken full-time 9am-6pm on weekdays, and on a part-time basis outwith those hours.
Read the rules here