Although it is September, the sun is still high in the sky and he sweats in his tweed suit, bow tie and stiff collar as he walks from the station. He’s mildly disgruntled having to return to London from his cottage at Barton Mills in Suffolk. He could be walking in the woods or along the banks of the gently flowing River Lark.
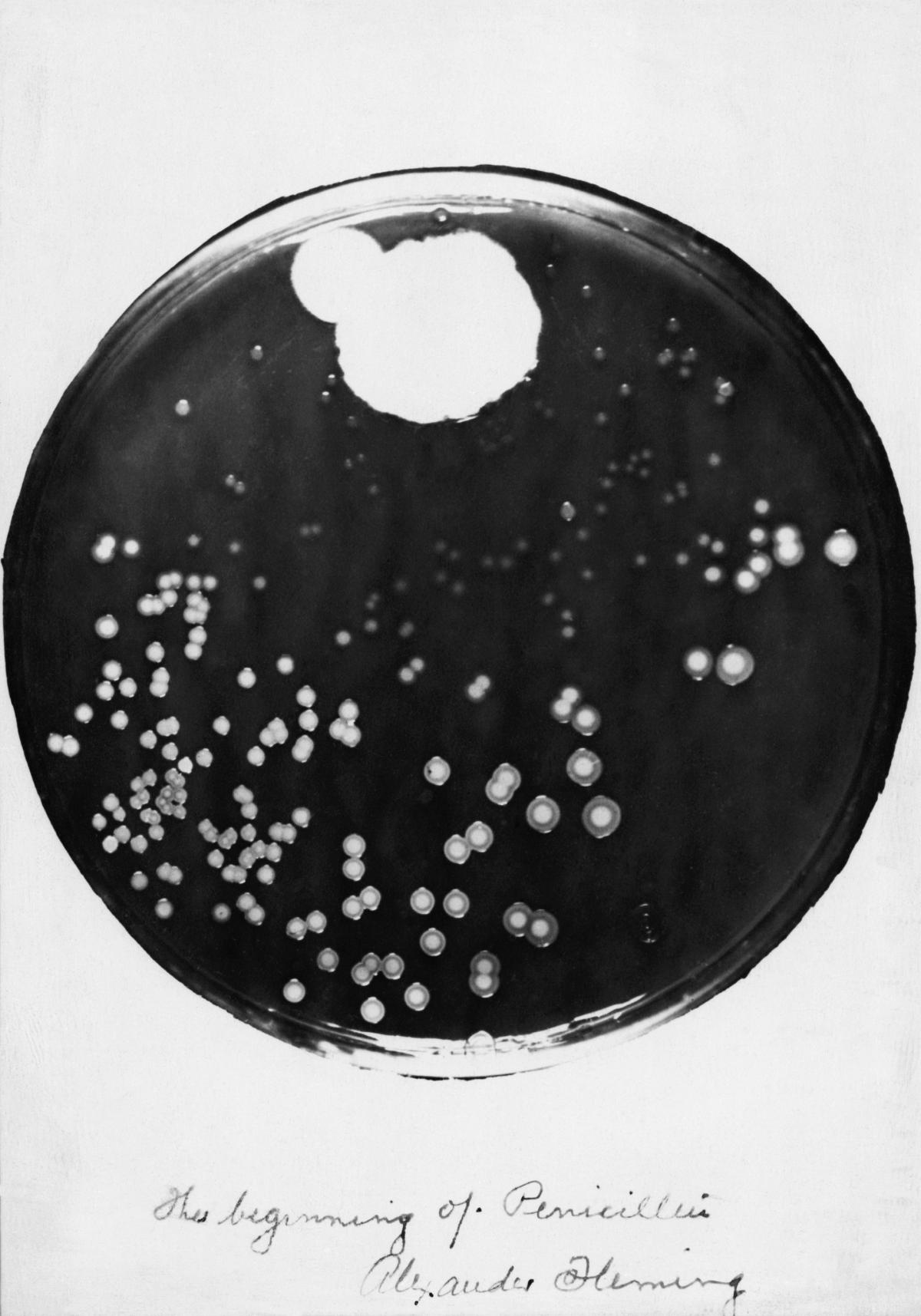
When he arrives at work he puts down his briefcase, takes off his jacket, and puts on a white laboratory coat. He isn’t the tidiest or most organised of men and tuts to himself and shakes his head to his assistant after noticing mould on a dish which has grown since he’s been on holiday.
He picks it up, studies it, peers at the dark green growth on it and the translucent ring around which seems to have dissolved the bacteria he was culturing.
He holds the dish up to the other scientist. “It’s funny,” he says. Then shakes his head, runs the dish under the hot tap and then drops it into a bucket of disinfectant.
He doesn’t know it but he has just washed away the chance to save hundreds of millions of lives, win a Nobel prize, gain a knighthood, and become one of the most famous and influential people of all time.
What if he had done just that in this counter-factual account of how Alexander Fleming failed to discover penicillin? We can’t know for sure how many lives the antibiotic has saved but medical historians reckon it could be 200 million or more.
It would not have been available to treat wounded British soldiers from D-Day onwards in the Second World War and it’s almost certain that other antibiotics, like the anti-TB drug, streptomycin, used today in open-heart and transplant surgery, wouldn’t be available.
But wouldn’t some other messy scientist have discovered it, just as someone else might have discovered gravity had Isaac Newton not decided to read a book under an apple tree in autumn? Almost certainly not.
The development of it and other antibiotics flows directly from Fleming’s accidental discovery at St Mary’s Hospital in London in 1928.
Fleming named it penicillin, after initially calling it mould juice. In February 1929, he presented his findings in a talk at the Medical Research Club. It passed almost unnoticed. Later in the year he wrote up his discovery for the British Journal of Experimental Pathology, again without any great impact.
As late as 1936, there was no interest in penicillin. When Fleming again presented his findings at an international microbiology summit in London, no-one believed him. In 1941, the British Medical Journal even wrote that it “does not appear to have been considered as possibly useful from any other point of view”.
Culture club
The difficulty was in purifying it and producing it in large amounts. A scientist at Oxford University, an emigré German Ernst Boris Chain, had read Fleming’s almost forgotten paper and was studying penicillin’s molecular structure. In 1940, he published his findings.
After reading it, Fleming phoned Howard Florey, head of development at the Oxford Group, to say he’d be visiting in the next few days. When Florey told Chain of the forthcoming visit, he replied: “Good God, I thought he was dead.”
Amazingly, a culture of Fleming’s mould was already kept at Oxford which enabled Chain to make an instant start on purifying penicillin. The rest is history: penicillin was purified and eventually produced in vast quantities, enough to help the Allies win the war (as well as clearing venereal infections).
Without Fleming’s discovery, Chain would have never chanced upon his paper and begun his work on penicillin.
When two Americans patented in the US the method of producing penicillin, it infuriated Fleming. He said: “I found penicillin and have given it free for the benefit of humanity. Why should it become a profit-making monopoly of manufacturers in another country?”
A comment that resonates today over the patenting of Covid vaccines.
There’s another “what if?” – Hitler’s doctor used penicillin to treat him after he was wounded in an assassination attempt in 1944. Had he not, he would probably have died and Germany might well have sued for peace.
Unlike Hitler, German soldiers did not have access to penicillin. There were limited supplies for British soldiers, which presented an ethical problem for doctors. Should it be given to the badly wounded or those with the clap? Winston Churchill reportedly decided it should be used to “best military advantage” – that is, to those with VD to get them back onto the frontlines more quickly.
Fleming was born on Lochfield farm, outside Darvel, in 1881, the seventh of eight children and educated at the local school then Kilmarnock Academy, before he moved to London at 16. He worked for four years in a shipping office and if his brother Tom, a doctor, hadn’t suggested he enrol at St Mary’s Medical School there would have been no penicillin, or antibiotics, which nearly all of us in the developed work have benefited from.
Counter-factually he may also have returned to Darvel to run the family farm (where there are self-catering cottages today).
Biographies of Fleming have suggested he lost interest in his discovery during the 1930s and if he had been more active in pursuing it then it would have been introduced earlier. This isn’t so. He may have been a mite disorganised – fortunately for us – but he was determined and indomitable.
A wish granted
A RESEARCH grant application to the Medical Research Council in 1930 is evidence of his continuing interest. The grant was for one of Fleming’s assistants to continue work on developing penicillin.
The grant was refused and, mysteriously, the page detailing the request has been excised from otherwise complete MRC records. Had the money come through it is possible that penicillin would have been produced a decade earlier, as presumably the person who had refused it realised and doctored the record.
Fleming’s interest in wound infection came in the First World War when he worked as a bacteriologist in a laboratory in Boulogne. More soldiers were dying from infected wounds than on the battlefield. They were being treated with strong antiseptics which Fleming demonstrated did more harm than good – he said that wounds should be treated instead with a mild saline solution. No-one listened.
Fleming’s first discovery, before his world-changing one, came in another fortuitous and sloppy manner. In 1921, he identified lysozyme, an enzyme with a mild antiseptic effect. He had a cold and a drop of his snot fell onto a dish culturing bacteria. He mixed the mucus into the culture and, weeks later, the bacteria had dissolved. It was an important indicator of how the body fights infection
From mucus he moved to tears, which had the same antiseptic effect. His research scholar at the time, VD Allison, wrote: “For the next five or six weeks, our tears were the source of supply for this extraordinary phenomenon. Many were the lemons we used (after the failure of onions) to produce a flow of tears ... The demand by us for tears was so great, that laboratory attendants were pressed into service, receiving threepence for each contribution.”
He went on to use blood, semen and egg white, proving that a bactericidal agent was present in all these. Like penicillin to come his findings were largely ignored by the medical establishment.
But it was penicillin that changed the world, from countering respiratory infections like pneumonia, the eradication of tuberculosis in developed countries to infected wounds.
Professor Christopher Tang, at the Sir William Dunn School of Pathology at Oxford University, puts it like this: “The sort of cancer chemotherapy which we currently use, which immunosuppresses people, we couldn’t possibly consider that without the use of antibiotics. So, not only has penicillin opened the door for treating people with infection, it’s also paved the way for modern medicine, modern interventional medicine that we benefit from now.”
Sir Alexander Fleming died on March 11 in 1955 and was buried at St Paul’s Cathedral. In the counter-factual account, the local farmer lies in an untended grave in Darvel cemetery.




Why are you making commenting on The Herald only available to subscribers?
It should have been a safe space for informed debate, somewhere for readers to discuss issues around the biggest stories of the day, but all too often the below the line comments on most websites have become bogged down by off-topic discussions and abuse.
heraldscotland.com is tackling this problem by allowing only subscribers to comment.
We are doing this to improve the experience for our loyal readers and we believe it will reduce the ability of trolls and troublemakers, who occasionally find their way onto our site, to abuse our journalists and readers. We also hope it will help the comments section fulfil its promise as a part of Scotland's conversation with itself.
We are lucky at The Herald. We are read by an informed, educated readership who can add their knowledge and insights to our stories.
That is invaluable.
We are making the subscriber-only change to support our valued readers, who tell us they don't want the site cluttered up with irrelevant comments, untruths and abuse.
In the past, the journalist’s job was to collect and distribute information to the audience. Technology means that readers can shape a discussion. We look forward to hearing from you on heraldscotland.com
Comments & Moderation
Readers’ comments: You are personally liable for the content of any comments you upload to this website, so please act responsibly. We do not pre-moderate or monitor readers’ comments appearing on our websites, but we do post-moderate in response to complaints we receive or otherwise when a potential problem comes to our attention. You can make a complaint by using the ‘report this post’ link . We may then apply our discretion under the user terms to amend or delete comments.
Post moderation is undertaken full-time 9am-6pm on weekdays, and on a part-time basis outwith those hours.
Read the rules hereLast Updated:
Report this comment Cancel